Get candid with the GEMTESA Go-Getters and hear how they’re standing up to OAB
Watch RealPatient Stories

Help us help you. Where are you in the OAB journey?
Treating OAB with GEMTESA has its benefits
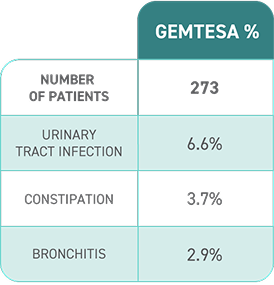
Proven results
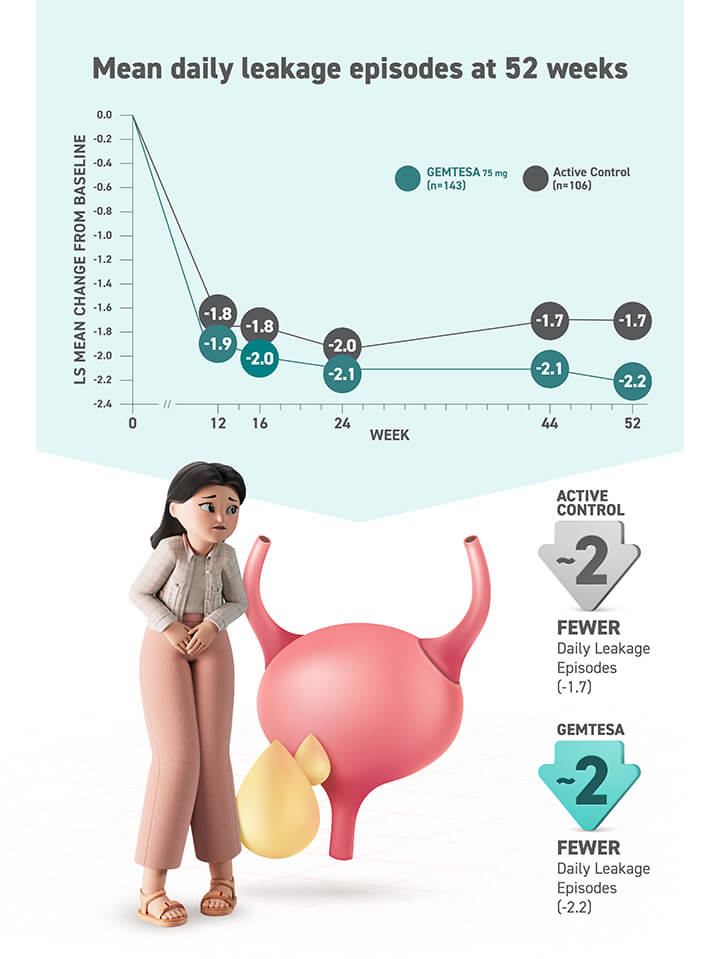
- Significant reductions seen in all 3 key OAB symptoms* vs placebo (sugar pill) at 12 weeks, including urgency
- Urgency episodes reduced by half in about 43% of people taking GEMTESA vs 38% of people taking placebo at 12 weeks
*Urgency, frequency, and leakage episodes.
A simple routine
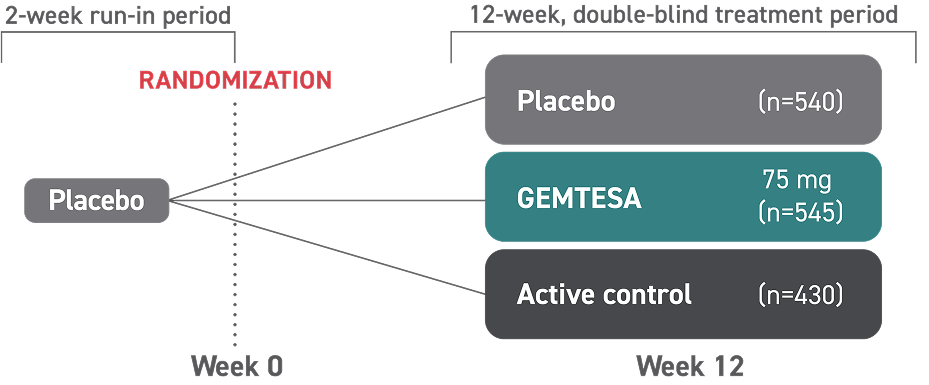
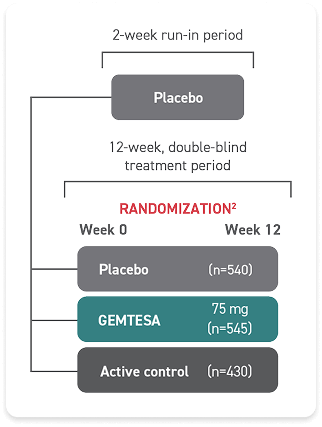
- One single-dose pill taken once daily with or without food
- GEMTESA tablets should be swallowed whole with a glass of water
- In adults, GEMTESA tablets may be crushed, mixed with a tablespoon (~15 mL) of applesauce, and taken immediately with a glass of water
No significant impact on blood pressure
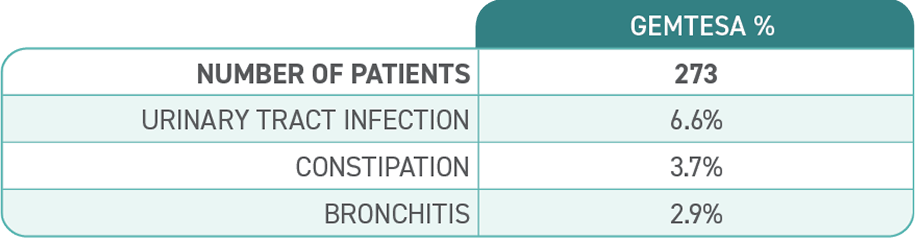
- No clinically significant changes to hypertension or increased blood pressure vs placebo†
†In a clinical study of men and women, people taking GEMTESA and those taking placebo had similar rates of hypertension (1.7% [9 out of 545 people] vs 1.7% [9 out of 540 people], respectively) and increased blood pressure (0.7% [4 out of 545 people] vs 0.9% [5 out of 540 people], respectively).
In a study of OAB in men being treated for benign prostatic hyperplasia (BPH), rates of hypertension were 9.0% with GEMTESA (50 of 553 people) vs 8.3% with placebo (46 of 551 people).