MORE
TIME
HERE
LESS
TIME
THERE

Help make a difference for overactive bladder (OAB) patients with GEMTESA2
Broadly Effective2
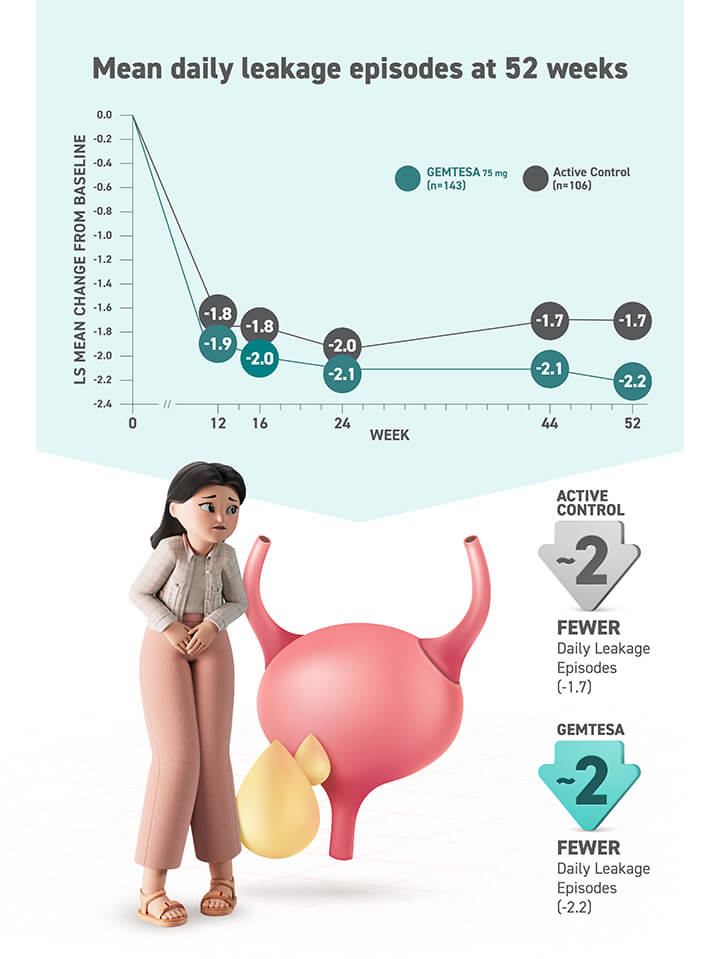
The ONLY β3 treatment with proven reduction data for all 3 key symptoms of OAB in its label2
- Urgency
- Frequency
- Urge urinary incontinence
Effective for a Wide Range of Patients2,4-6
- Women and men, regardless of prior therapy
- Younger and older adults (65+)
- Patients with hypertension*
- Patients taking medicines metabolized by the CYP2D6 pathway
- Male patients with overactive bladder being pharmacologically treated for benign prostatic hyperplasia
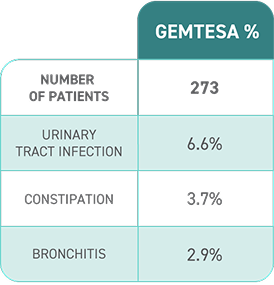
Established Safety
in OAB2,4,7
- The ONLY β3 treatment with no blood pressure warning in its label
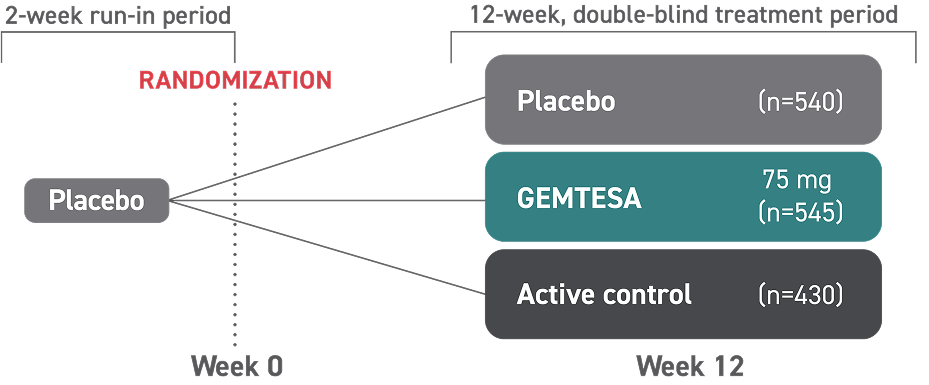
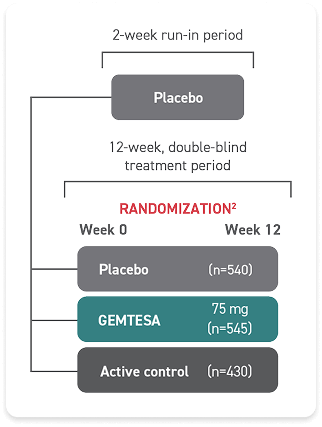
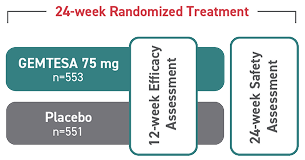
- In a 24-week study of OAB in men being treated for BPH, rates of hypertension were 9.0% with GEMTESA (n=553) vs 8.3% with placebo (n=551)2
- The ONLY β3 treatment with no drug interaction with CYP2D6 substrates used for common comorbidities2,6
Access GEMTESA for Commercial and Medicare Part D patients
We’re committed to helping more patients with access support and resources.
Concomitant use of GEMTESA increases digoxin maximal concentrations (Cmax) and systemic exposure as assessed by area under the concentration-time curve. Serum digoxin concentrations should be monitored before initiating and during therapy with GEMTESA and used for titration of the digoxin dose to obtain the desired clinical effect. Continue monitoring digoxin concentrations upon discontinuation of GEMTESA and adjust digoxin dose as needed.2
*In a 4-week, randomized, placebo-controlled, ambulatory BP study in OAB patients (n=200), daily treatment with GEMTESA 75 mg was not associated with clinically significant changes in BP. Subjects enrolled in this study had a mean age of 59 years and 75% were female. Thirty-five percent of subjects had preexisting hypertension at baseline and 29% of all subjects were taking at least 1 concomitant antihypertensive medication.2,7
AUA=American Urological Association; BP=blood pressure; BPH=benign prostatic hyperplasia; SUFU=Society of Urodynamics, Female Pelvic Medicine & Urogenital Reconstruction.