The GEMTESA pivotal study had more than 1500 patients with OAB and the extension study had over 500 patients1

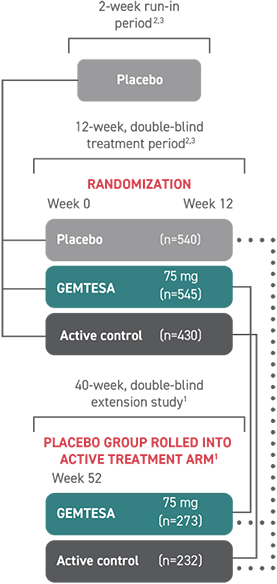
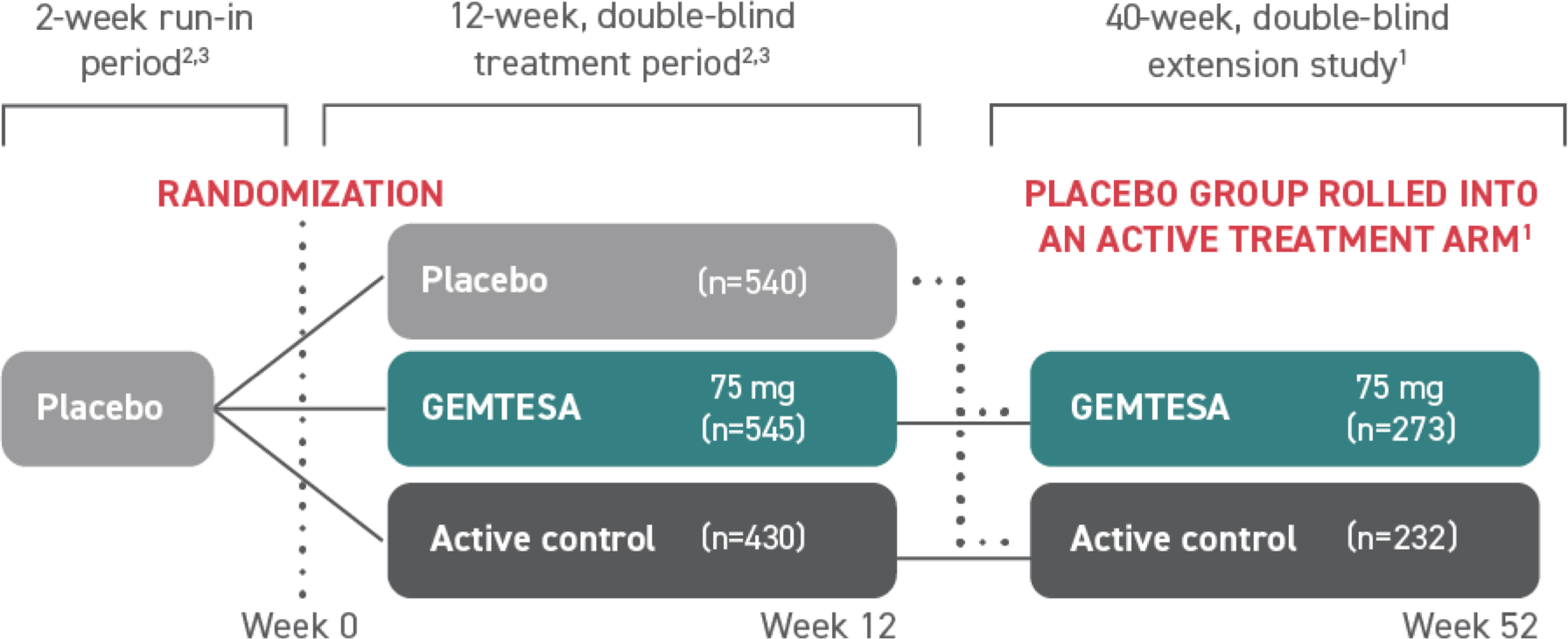
GEMTESA (vibegron) was studied in a phase 3, randomized, double-blind, placebo- and active-controlled multicenter study2,3

At Week 12, safety and efficacy were evaluated in over 1500 patients with symptoms of overactive bladder (OAB)2,3
- GEMTESA demonstrated a statistically significant reduction of all 3 key OAB symptoms* vs placebo at 12 weeks—including urgency2,4†
- ~43% of all OAB patients taking GEMTESA had a 50% reduction in urgency episodes at Week 12 vs 38% with placebo3‡

505 patients continued on to a 40-week, phase 3, randomized, double-blind, active-controlled, multicenter extension study that evaluated the safety and efficacy of GEMTESA1§
*The 3 key symptoms of OAB are urgency, micturition frequency, and urge urinary incontinence (UUI).4
†The efficacy of GEMTESA was evaluated in a 12-week, double-blind, randomized, placebo- and active-controlled trial in patients with OAB (UUI, urgency, and urinary frequency). For study entry, patients had to have symptoms of OAB for at least 3 months with an average of 8 or more micturitions per day and at least 1 UUI per day, or an average of 8 or more micturitions per day and an average of at least 3 urgency episodes per day. A total of 1515 patients received at least 1 daily dose of placebo (n=540), GEMTESA 75 mg (n=545), or an active-control treatment (n=430). The majority of patients were Caucasian (78%) and female (85%), with a mean age of 60 (range 18 to 93) years.2,3
‡Data were based on unadjusted values for a supportive outcome measure that was a prespecified secondary endpoint in the pivotal EMPOWUR trial.3
§A limitation of this study is the potential for selection bias for patients who elected to enter the EMPOWUR extension.1
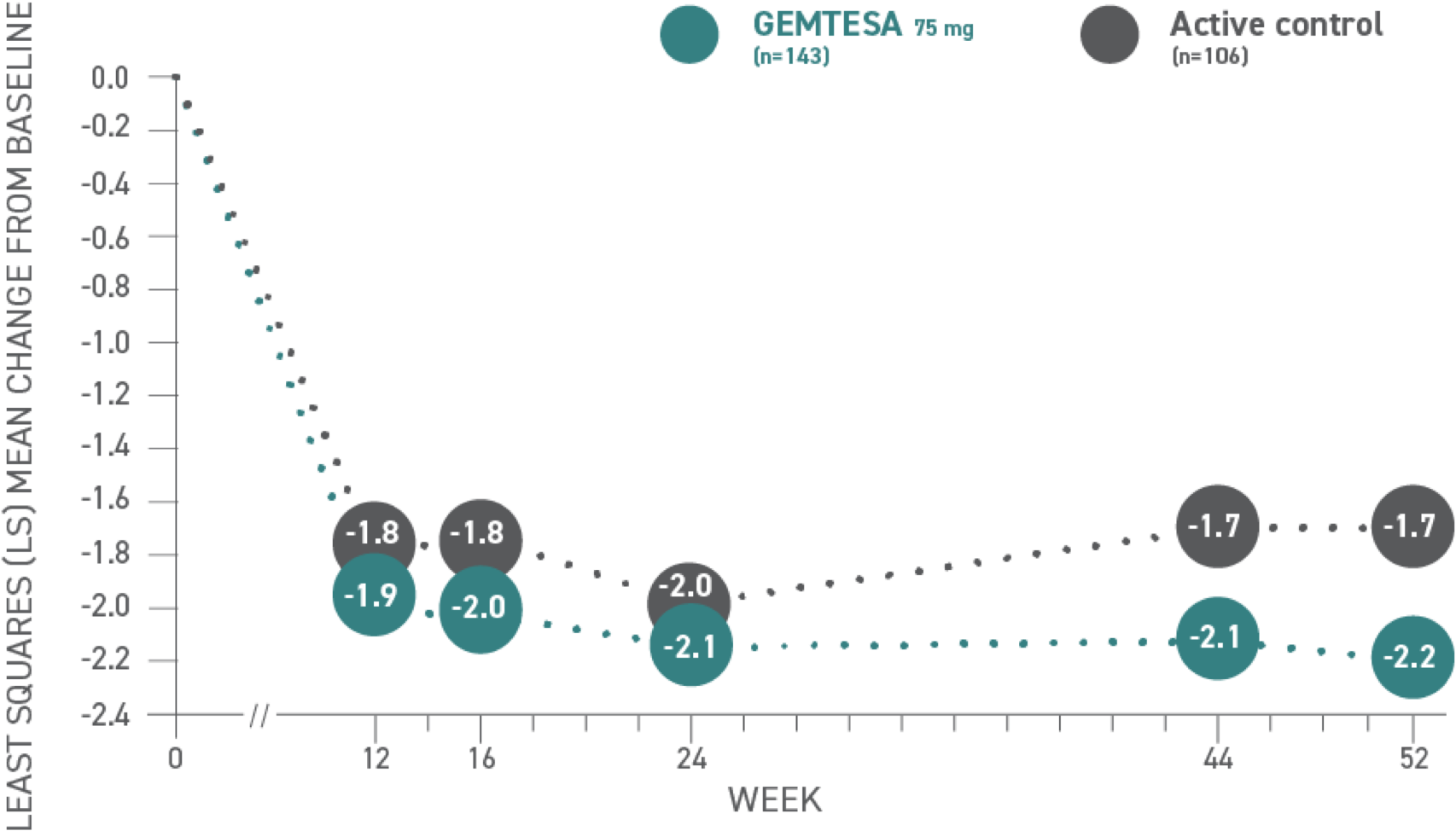
Safety and efficacy of GEMTESA were evaluated at 52 weeks1
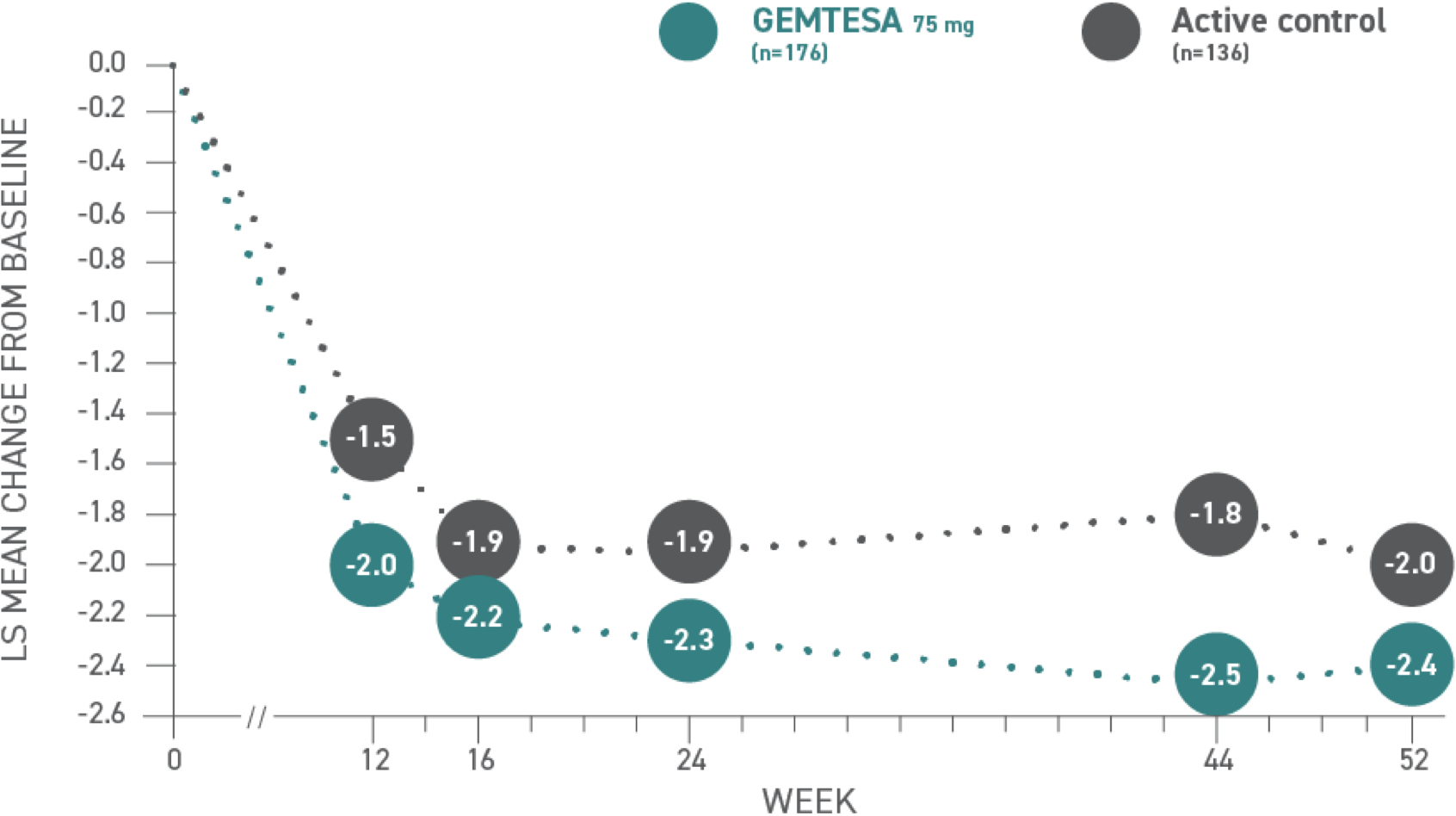
Reductions in UUI per 24 hours at 52 weeks vs active control1,3*
*Baseline: 3.18 for GEMTESA; 3.0 for active control.3
Urge urinary incontinence (UUI)1,3*
Values
| Week | 2 | 4 | 8 | 12 |
|---|---|---|---|---|
| Placebo (n=405) | -0.8 | -1.0 | -1.2 | -1.4 |
| GEMTESA 75mg (n=403) | -1.4 | -1.7 | -1.8 | -2.0 |
*Baseline: 3.18 for GEMTESA; 3.0 for active control.3
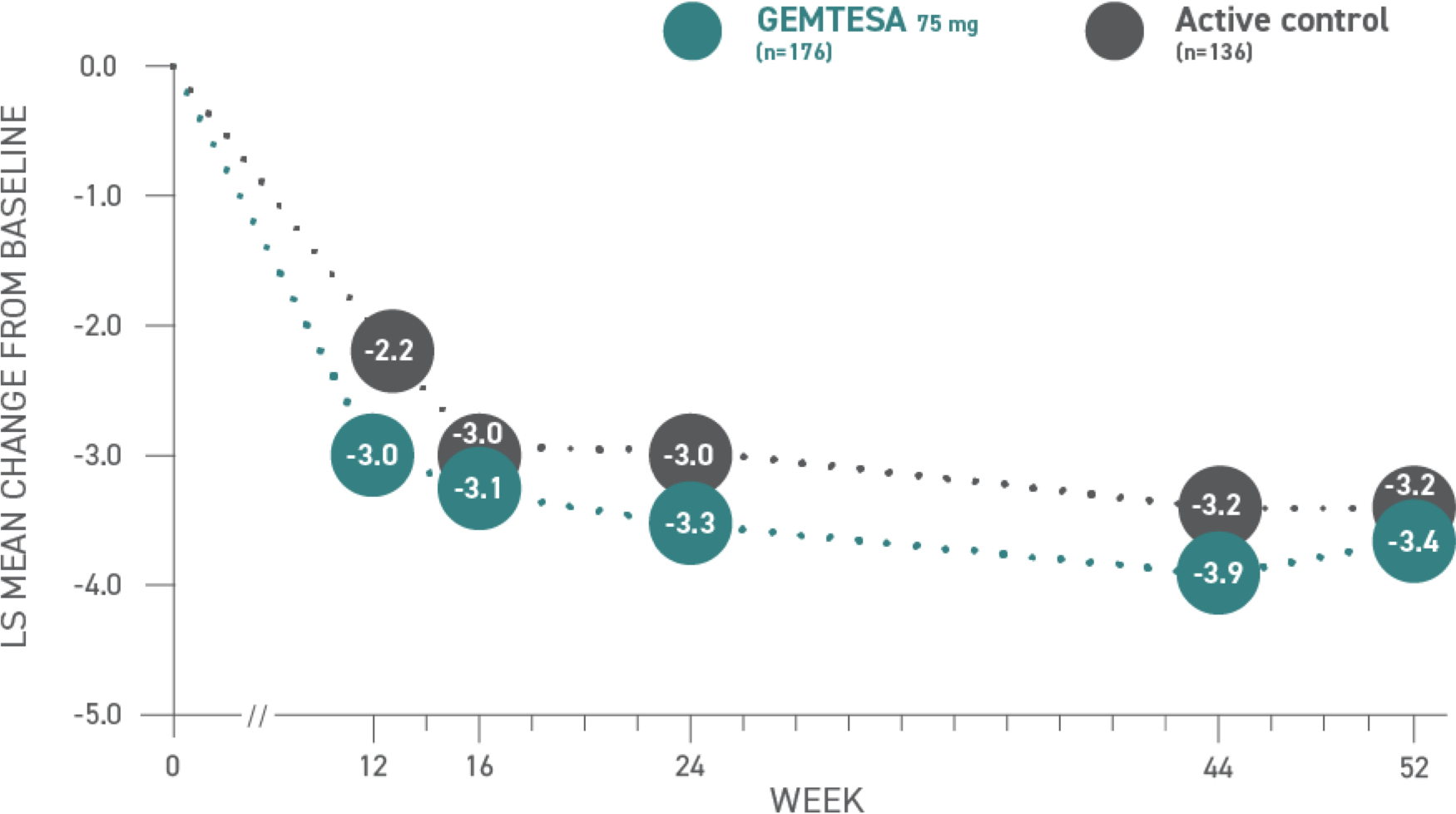
Reductions in micturition frequency per 24 hours at 52 weeks vs active control1*
Please see extension study design above.
Micturition frequency1,3*
Values
| Week | 2 | 4 | 8 | 12 |
|---|---|---|---|---|
| Placebo (n=520) | -0.5 | -0.7 | -1.0 | -1.3 |
| GEMTESA 75mg (n=526) | -0.9 | -1.2 | -1.6 | -1.8 |
Reductions in micturition frequency per 24 hours at 52 weeks vs active control1*
*Baseline: 11.32 for GEMTESA; 11.33 for active control.3
Please see extension study design above.
Reductions in urgency episodes
per 24 hours at 52 weeks vs
active control1*
*Baseline: 8.0 for GEMTESA; 8.03 for active control.3
Please see extension study design above.
Urgency episodes1,3*
Values
| Week | 2 | 4 | 8 | 12 |
|---|---|---|---|---|
| Placebo (n=520) | -0.9 | -1.1 | -1.6 | -2.0 |
| GEMTESA 75mg (n=526) | -1.5 | -1.9 | -2.4 | -2.7 |
*Baseline: 8.0 for GEMTESA; 8.03 for active control.3
Please see extension study design above.
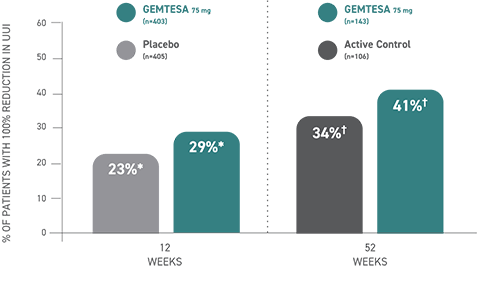
100% reduction in UUI episodes1,3
Values
| 12 Weeks | |
|---|---|
| Placebo (n=405) | 36.8% |
| GEMTESA 75mg (n=403) | 52.4% |
*Data were based on a key secondary endpoint analysis of OAB-wet patients with 100% reduction in UUI from baseline at Week 12 (n=403).3
†Data were based on an exploratory endpoint analysis of OAB-wet patients with 100% reduction in UUI from baseline at Week 52 (n=143).3
Please see extension study design above.