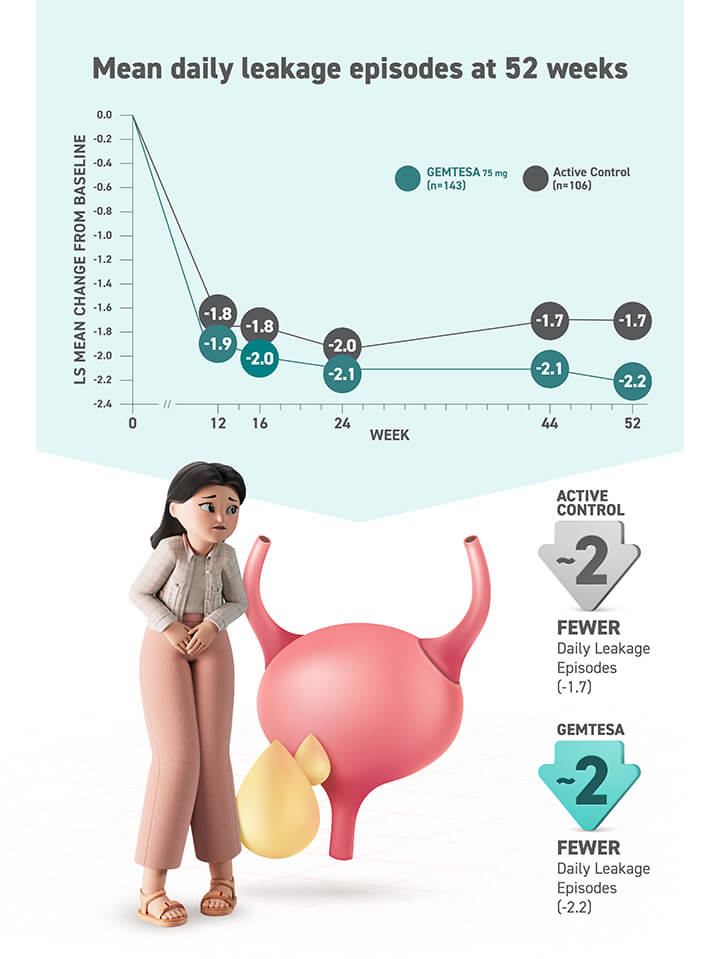
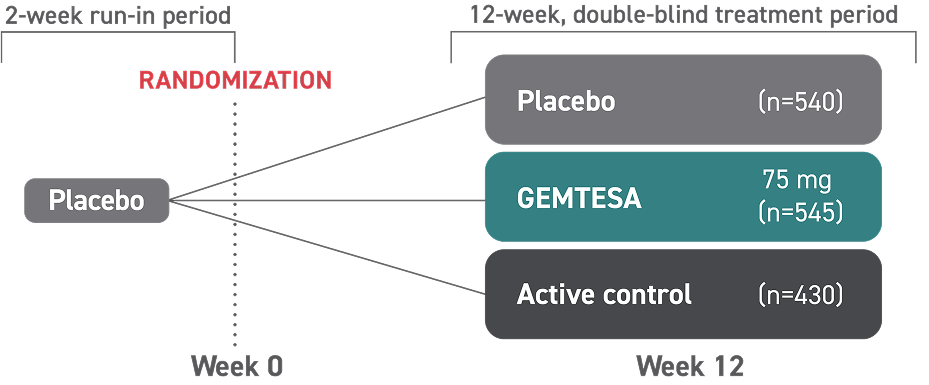
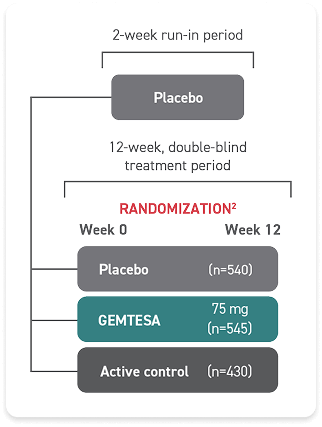
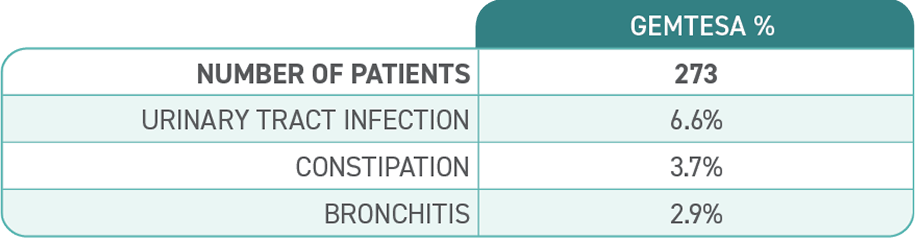
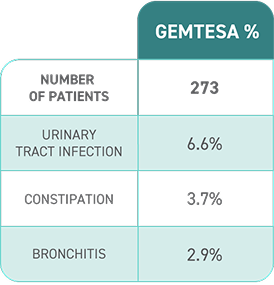
Access GEMTESA for your patients

Unrestricted access for the majority of patients with OAB nationwide1*
Commercially Insured Patients May Save With the GEMTESA Simple Savings Program1†
For maximum savings
ELIGIBLE PATIENTS
MAY PAY AS LITTLE AS
Eligible patients may pay as little as $10 a month for each covered 30-day prescription
Eligible patients whose insurance does not cover GEMTESA may pay as little as $95 a month
Costs for Medicare Part D Prescriptions May Be Lower and More Predictable2-5‡§
Lower annual patient out-of-pocket (oOP) max
$2,000 for all covered drugsIf patient reaches OOP max
$0 CopayMedicare Prescription Payment Plan
Patient can opt in to split OOP costs into monthly installments*All formulary data and access criteria are provided by the Managed Markets Insights & Technology, LLC, database as of March 2025.1
†Restrictions and maximum saving limits apply. Offers not valid for patients participating in Medicare, Medicaid, or other government healthcare programs. Programs are subject to change. See full Terms, Conditions, and Eligibility Criteria at GEMTESA.com/card.
‡Time frame to reach the $2,000 OOP maximum and begin paying $0 for covered prescriptions depends on individual plan benefits and monthly medication costs.
§Monthly payments may vary based on individual plan design and when the patient opts in to the Medicare Prescription Payment Plan.
OAB=overactive bladder.