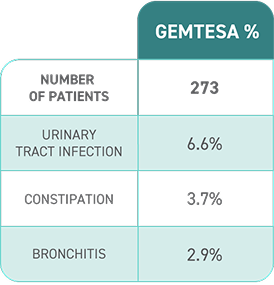
DISCOVER SAVINGS AND SUPPORT WITH GEMTESA
For a limited time, the GEMTESA SIMPLE SAVINGS PROGRAM* is providing commercially insured patients with a savings offer to lower their out of pocket costs.
Choose from 2 ways to sign up:
Save with the GEMTESA Simple Savings Card
Text† “GEMTESA” to 436872
- Complete the enrollment process
- If eligible, your savings card information will be texted to your phone. Bring this information to the pharmacy when picking up your GEMTESA prescription
Sign up today to take advantage of support resources including easy and convenient refill reminders
*Restrictions and maximum saving limits apply. Coverage and out-of-pocket costs may vary. Offer not valid for patients participating in Medicare, Medicaid, or other government healthcare programs. See full Program Terms, Conditions, and Eligibility Criteria.
†Message and Data rates may apply. Up to 15 messages per month after initial sign-up communications. Text STOP to unsubscribe at any time.

See how much eligible patients could save on GEMTESA
LIMITED TIME OFFER THROUGH DECEMBER 31, 2026
You may
pay as little as
$0
for each 90-day
supply if*:
- You have commercial insurance (government plans like Medicare or Medicaid are not eligible)
- GEMTESA is covered by your plan
You may pay as little as
$10 for each 30-day supply if*:
- You have commercial insurance (government plans like Medicare or Medicaid are not eligible)
- GEMTESA is covered by your plan
You may pay as little as
$95 for each 30-day supply if*:
- You have commercial insurance (government plans like Medicare or Medicaid are not eligible)
- GEMTESA is not covered by your plan
The Wholesale Acquisition Cost (WAC), also known as the list price, for a 30-day supply of GEMTESA® (vibegron) 75 mg tablets is $500.89‡ as of August 2024. This is the price for products sold to wholesalers before any discount, rebate, chargeback, or other price reduction and therefore does not represent actual transaction prices. Your out-of-pocket cost will depend on your insurance coverage and savings program eligibility.
For questions about this program, please call 1-833-876-8268.
*Restrictions and maximum saving limits apply. Coverage and out-of-pocket costs may vary. Offer not valid for patients participating in Medicare, Medicaid, or other government healthcare programs. See full Program Terms, Conditions, and Eligibility Criteria.
‡The pricing information listed does not imply safety or efficacy of the product and is not typically associated with per-dose pricing, price per course of treatment, or the cost effectiveness of the products listed. No comparisons should be made.
Mail-in rebate option
- If you fill your prescription through a mail order pharmacy, or are unable to process your savings card at your local pharmacy, you will need the following items to request reimbursement:
- Photocopy of the front and back of your GEMTESA Simple Savings Card
- Original proof of purchase (original pharmacy receipt with your name, address, pharmacy name, product name, prescription numbers, NDC number, date filled, quantity, and price)
- Photocopy of the front and back of your insurance card
- Your date of birth
- Your mailing address
- Submit the items listed above via email to gemtesa@ApolloCare.com
- Please allow 6-8 weeks to receive reimbursement. Reimbursement requests must be postmarked within 3 months of the fill date. Reimbursements are subject to Program Terms, Conditions, and Eligibility Criteria