DISCOVER THE BENEFITS OF GEMTESA FOR LONG-TERM CARE (LTC) RESIDENTS WITH OVERACTIVE BLADDER (OAB)
GEMTESA (vibegron) offers symptom improvement, simple dosing, and a demonstrated safety and tolerability profile for patients with OAB1
No overall differences in safety or effectiveness of GEMTESA have been observed between patients aged 65+ and younger adult patients2
PROVEN EFFICACY IN OLDER ADULTS
- First and only beta-3 agonist with proven urgency reduction data in its label1
- Statistically significant reduction in all 3 key OAB symptoms* vs placebo at 12 weeks—including urgency1,3†
- Reductions in 3 key OAB symptoms* at 12 weeks in patients aged 65+ and 75+2
ONE CRUSHABLE DOSE
- First and only beta-3 agonist with a once-daily 75-mg dose with no titration, to be taken with or without food, and swallowed whole with a glass of water1
- Crushability. In adults, GEMTESA tablets may be crushed, mixed with a tablespoon (~15 mL) of applesauce and taken immediately with a glass of water1
ESTABLISHED SAFETY FOR OLDER ADULTS
- First and only beta-3 agonist with no blood pressure warning in its label1
- First and only beta-3 agonist with no drug interaction with CYP2D6 substrates (medications commonly taken by LTC residents)1
- Digoxin drug interaction was identified with GEMTESA. Measure serum digoxin concentrations before initiating GEMTESA. Monitor serum digoxin concentrations to titrate digoxin dose to desired clinical effect. Continue monitoring digoxin concentration upon discontinuation of GEMTESA and adjust digoxin dose as needed1
- No known association with cognitive decline, including dementia, for the beta-3 agonist class4,5
*The 3 key symptoms of OAB are urgency, micturition frequency, and urge urinary incontinence (UUI)/leakage.3
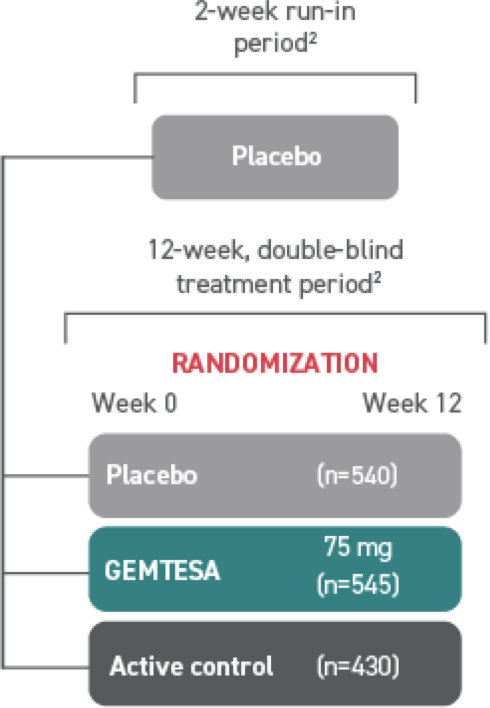
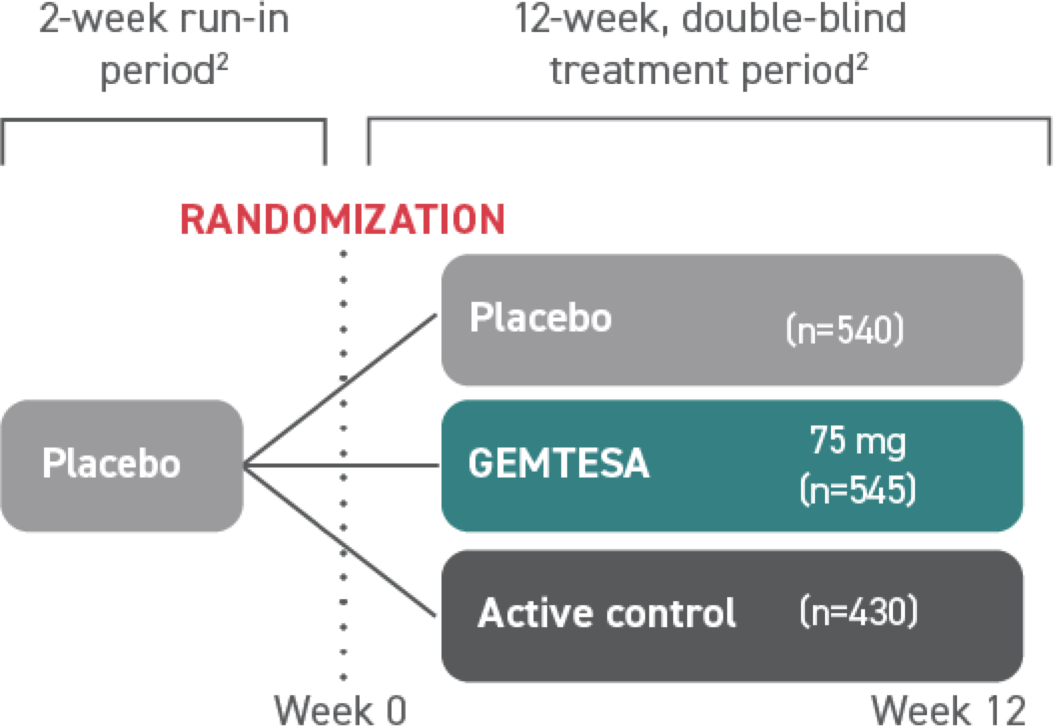
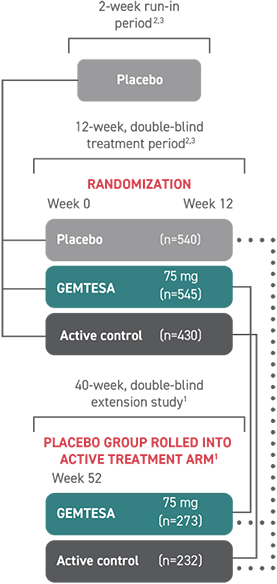
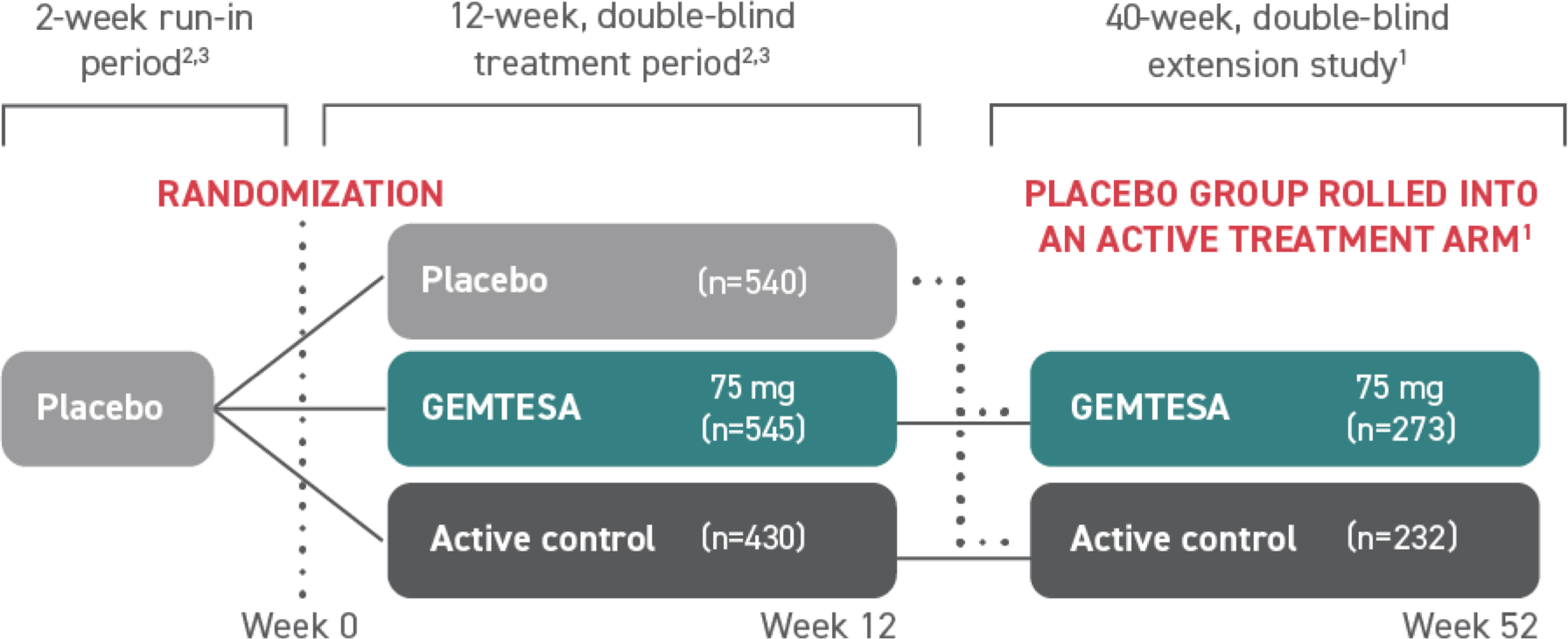
†The efficacy of GEMTESA was evaluated in a pivotal 12-week, double-blind, randomized, placebo- and active-controlled trial in patients with OAB (UUI, urgency, and urinary frequency). For study entry, patients had to have symptoms of OAB for at least 3 months, with an average of 8 or more micturitions per day and at least 1 UUI per day, or an average of 8 or more micturitions per day and an average of at least 3 urgency episodes per day. A total of 1515 patients received at least 1 daily dose of placebo (n=540), GEMTESA 75 mg (n=545), or an active-control treatment (n=430). The majority of patients were Caucasian (78%) and female (85%) with a mean age of 60 (range 18 to 93) years.1,5
GEMTESA was evaluated at 12 weeks and 52 weeks in the overall study population1,6,7
(pivotal study)
(extension study)
(n=403)
-2.0*
(n=405)
-1.4
(n=143)
-2.2
(n=106)
-1.7
(n=526)
-1.8†
(n=520)
-1.3
(n=176)
-2.4
(n=136)
-2.0
(n=526)
-2.7‡
(n=520)
-2.0
(n=176)
-3.4
(n=136)
-3.2
(n=403)
28.8%
(n=405)
22.5%
(n=143)
40.8%
(n=106)
34.2%
Efficacy analyses were performed using the FAS-EXT, except for incontinence episode endpoints, which used the FAS-EXT-I.7
*P<0.0001 vs placebo.1
†P<0.001 vs placebo.1
‡P<0.002 vs placebo.1
§Data were based on an exploratory endpoint analysis of patients with 100% reduction in UUI from baseline at week 52 (n=143).7
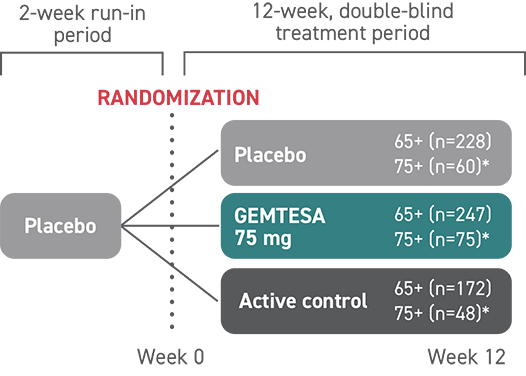
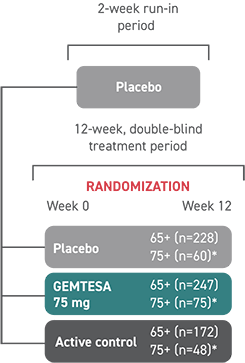
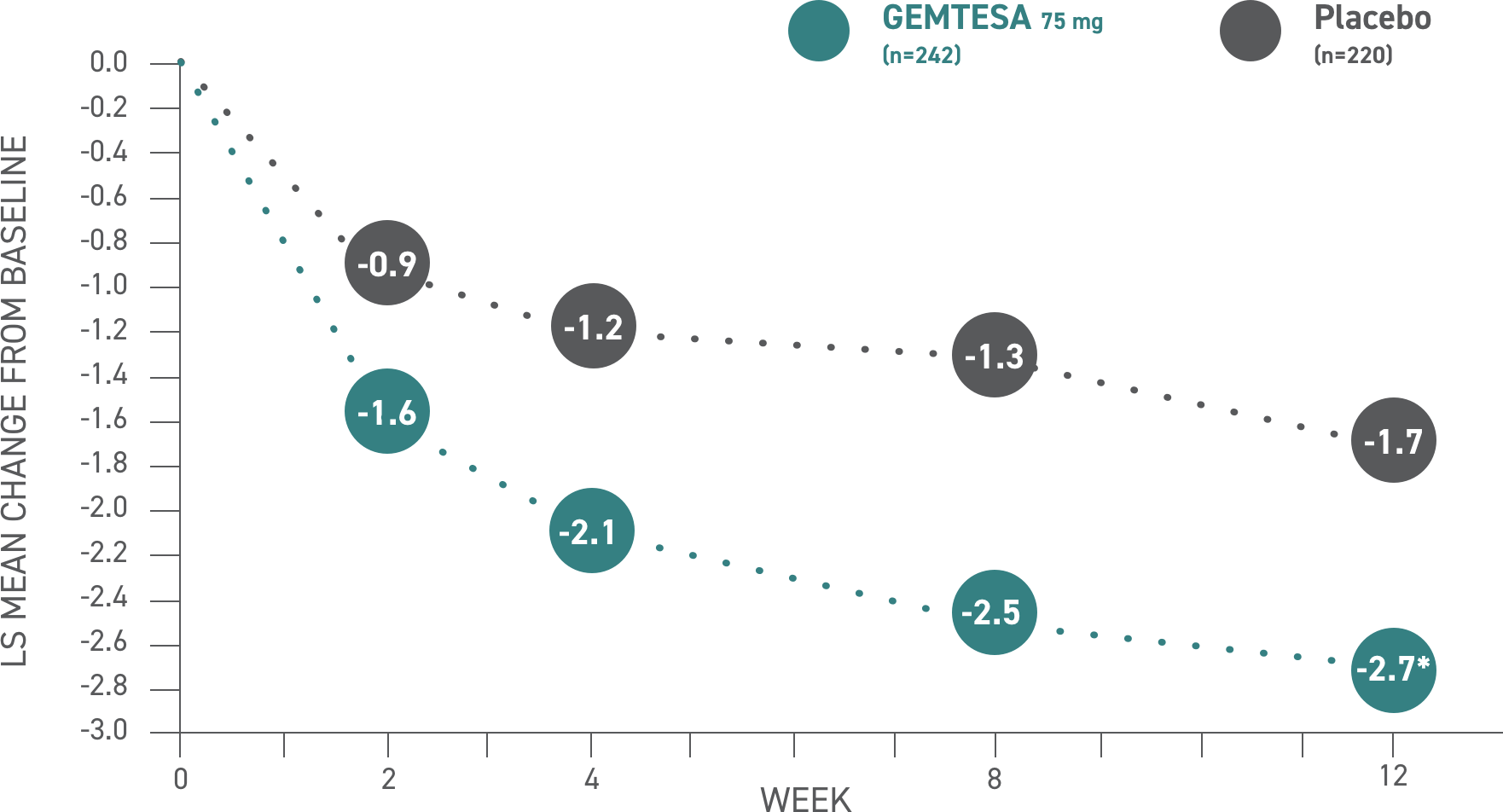
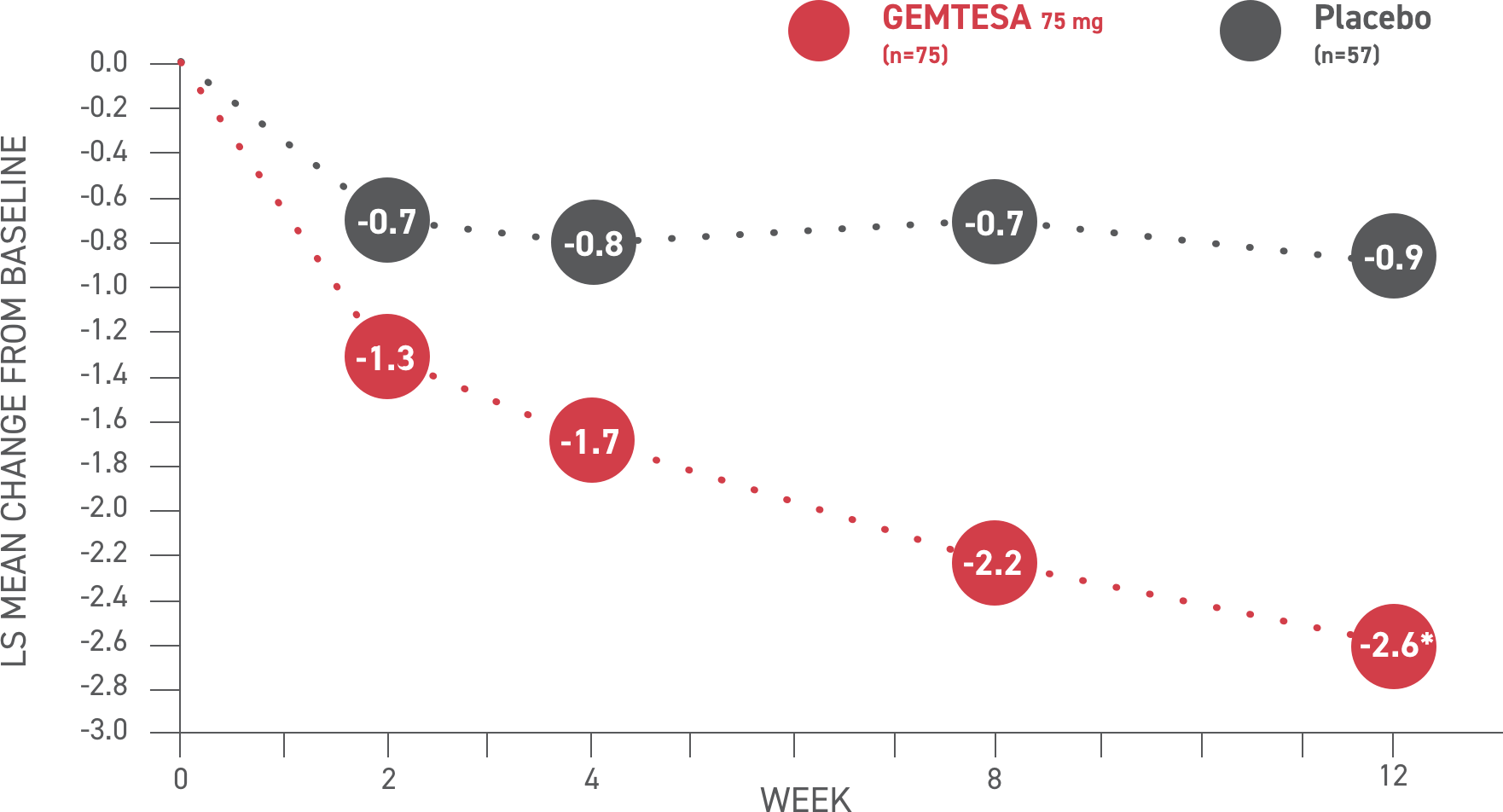
In the older adult subpopulation, reductions in all 3 key OAB symptoms* were consistent with the overall study population at 12 weeks1-3
No overall differences in safety or effectiveness of GEMTESA have been observed between patients aged 65+ and younger adult patients2
This post hoc subpopulation analysis was not powered to detect differences within subgroups and is therefore limited by the small sample sizes, particularly in the subgroup of patients aged ≥75 years
GEMTESA demonstrated significant reductions in average daily UUI episodes at 12 weeks (co-primary endpoint) vs placebo2†
*The 3 key symptoms of OAB are urgency, micturition frequency, and UUI/leakage.3
†P<0.001 vs placebo.2
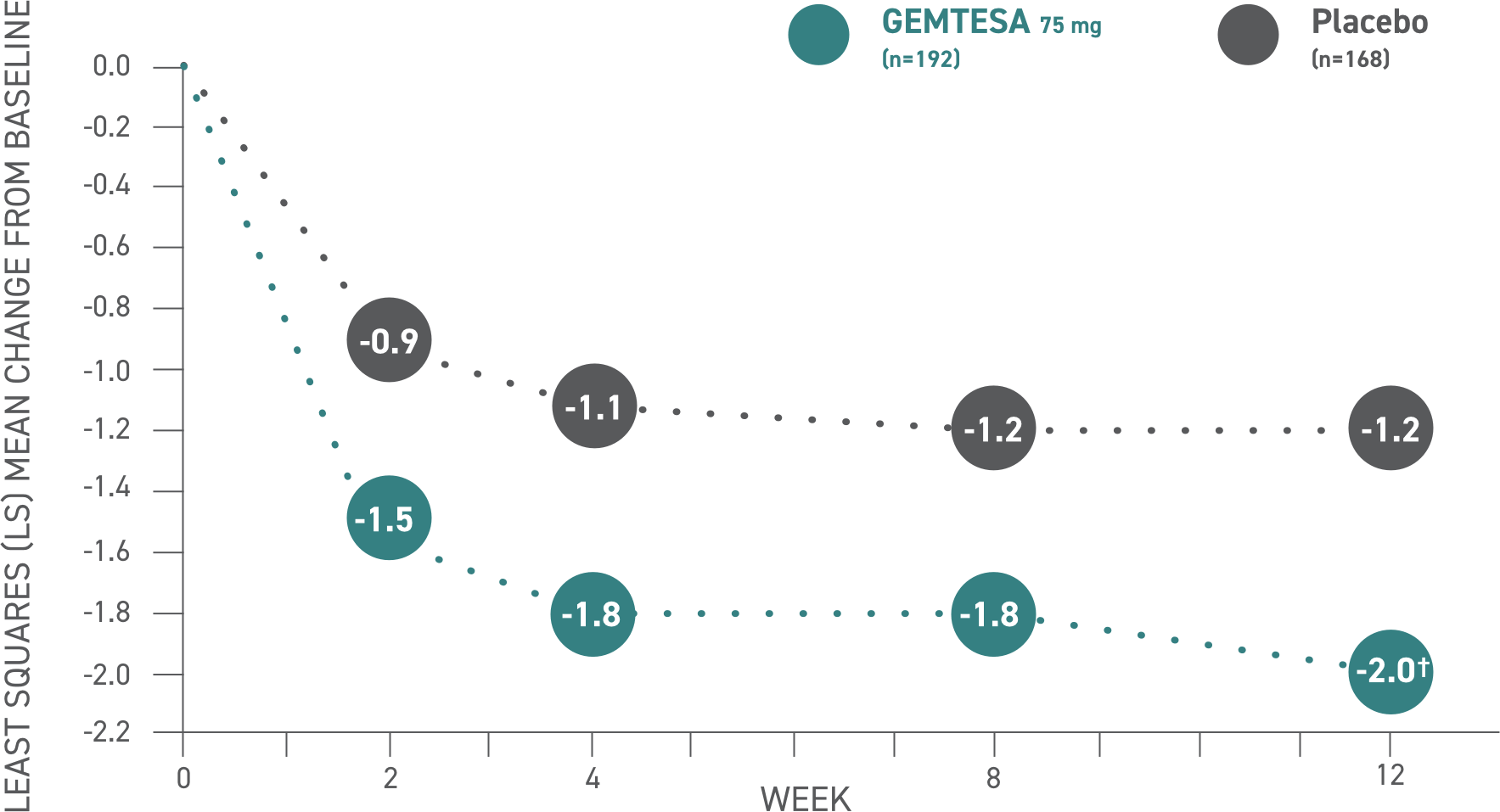
Urge urinary incontinence (UUI)2
Values
| Week | 2 | 4 | 8 | 12 |
|---|---|---|---|---|
| Placebo (n=405) | -0.8 | -1.0 | -1.2 | -1.4 |
| GEMTESA 75mg (n=403) | -1.4 | -1.7 | -1.8 | -2.0 |
GEMTESA demonstrated significant reductions in average daily UUI episodes at 12 weeks (co-primary endpoint) vs placebo2*
*P<0.001 vs placebo.
Please see older adults data analysis above.
(UUI)2
Values
| Week | 2 | 4 | 8 | 12 |
|---|---|---|---|---|
| Placebo (n=405) | -0.8 | -1.0 | -1.2 | -1.4 |
| GEMTESA 75mg (n=403) | -1.4 | -1.7 | -1.8 | -2.0 |
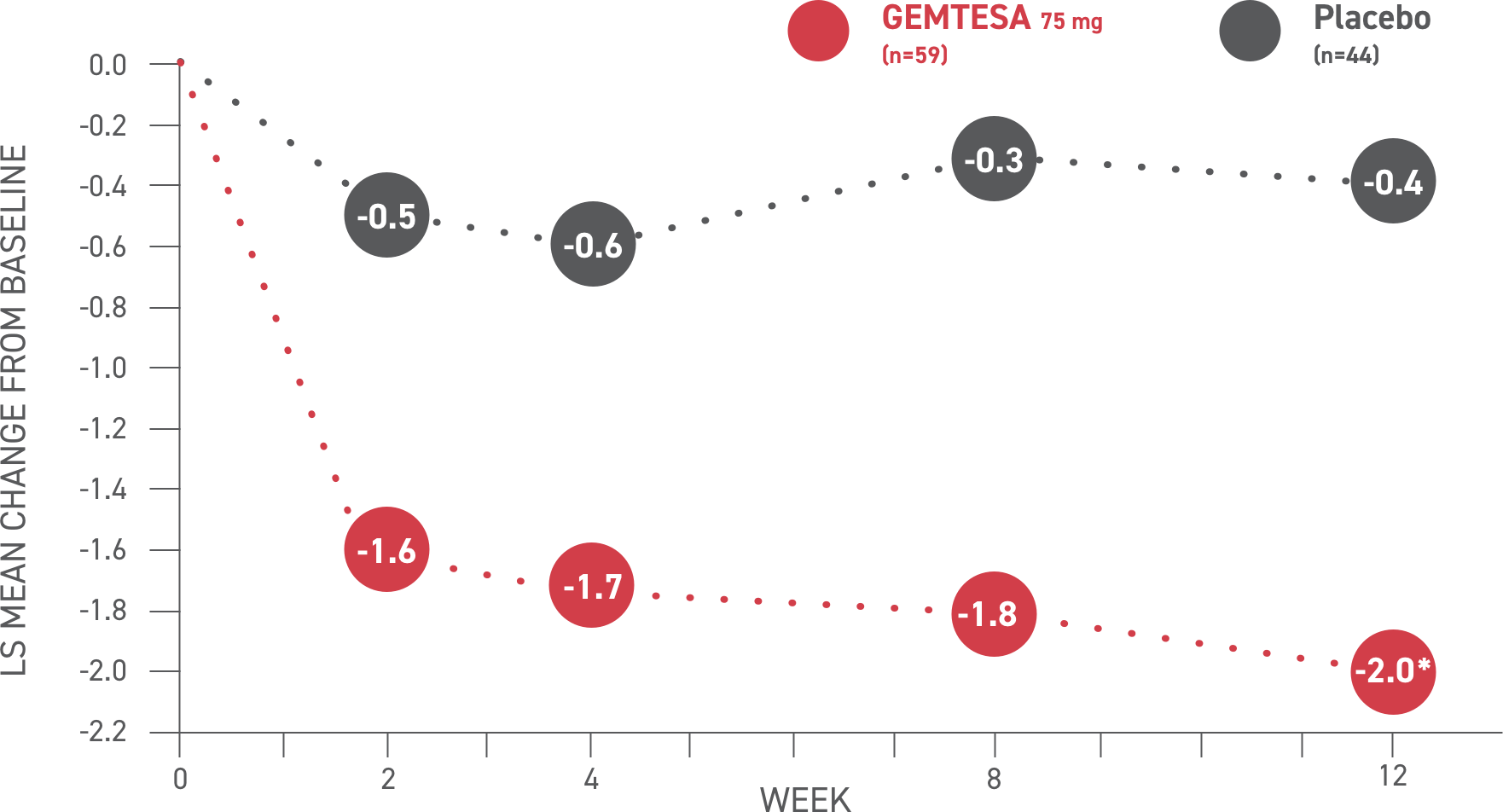
GEMTESA demonstrated significant reductions in average daily micturition frequency at 12 weeks (co-primary endpoint) vs placebo2*
*P<0.001 vs placebo.
Please see older adults data analysis above.
Micturition frequency2
Values
| Week | 2 | 4 | 8 | 12 |
|---|---|---|---|---|
| Placebo (n=405) | -0.8 | -1.0 | -1.2 | -1.4 |
| GEMTESA 75mg (n=403) | -1.4 | -1.7 | -1.8 | -2.0 |
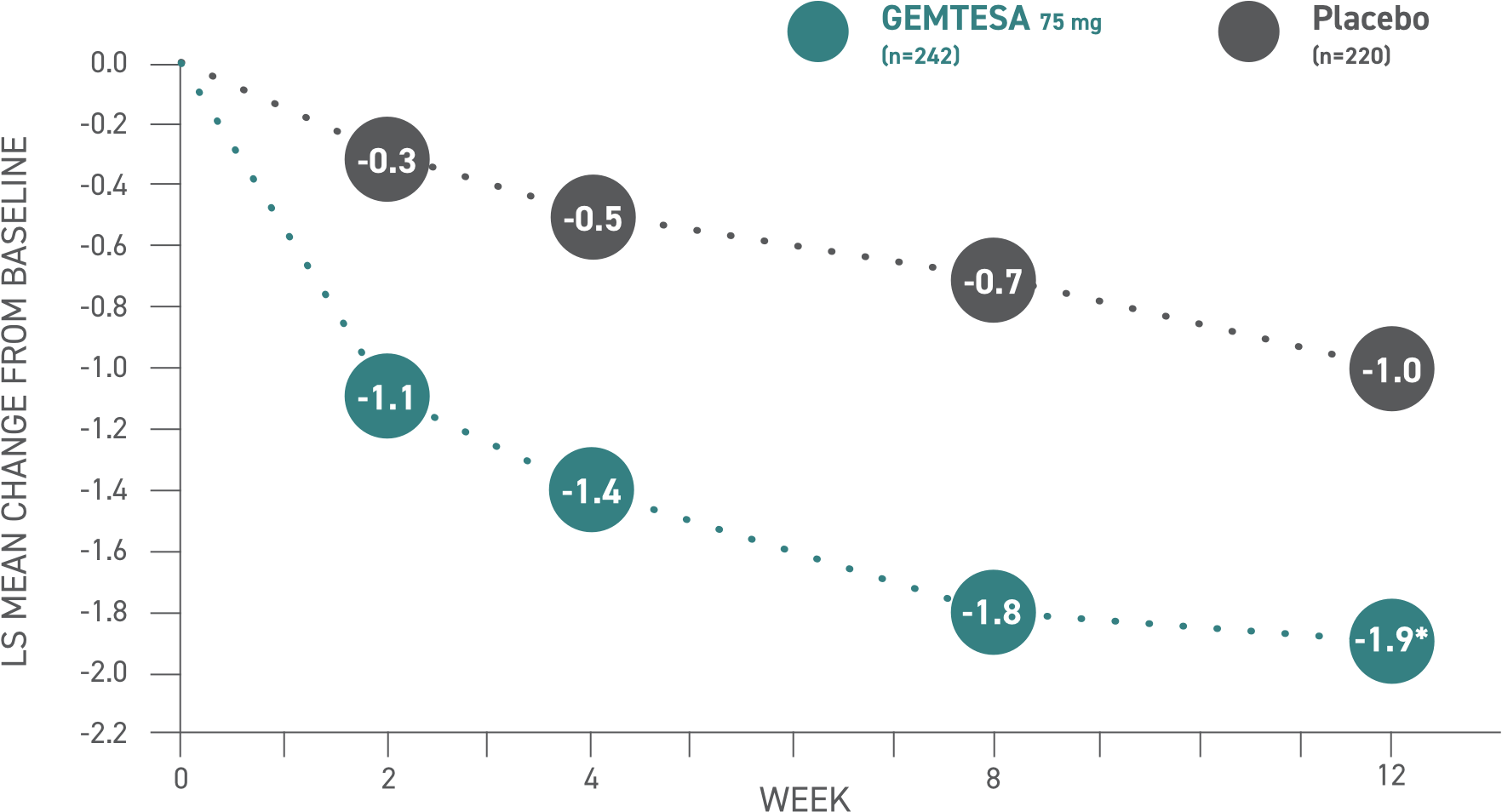
GEMTESA demonstrated significant reductions in average daily micturition frequency at 12 weeks (co-primary endpoint) vs placebo2*
*P<0.05 vs placebo.
Please see older adults data analysis above.
Micturition frequency2
Values
| Week | 2 | 4 | 8 | 12 |
|---|---|---|---|---|
| Placebo (n=405) | -0.8 | -1.0 | -1.2 | -1.4 |
| GEMTESA 75mg (n=403) | -1.4 | -1.7 | -1.8 | -2.0 |
Urgency is the hallmark OAB symptom8,9
While urgency is a critical factor in the diagnosis of OAB, the symptom often remains unreported and undiagnosed8,9
In a recently published study:
- Urgency progressed in severity in older patients
- Identifying and treating urgency in female individuals with lower urinary tract symptoms could help delay or possibly prevent progression as they age8
GEMTESA IS THE FIRST AND ONLY BETA-3 AGONIST WITH URGENCY REDUCTION DATA IN ITS LABEL1
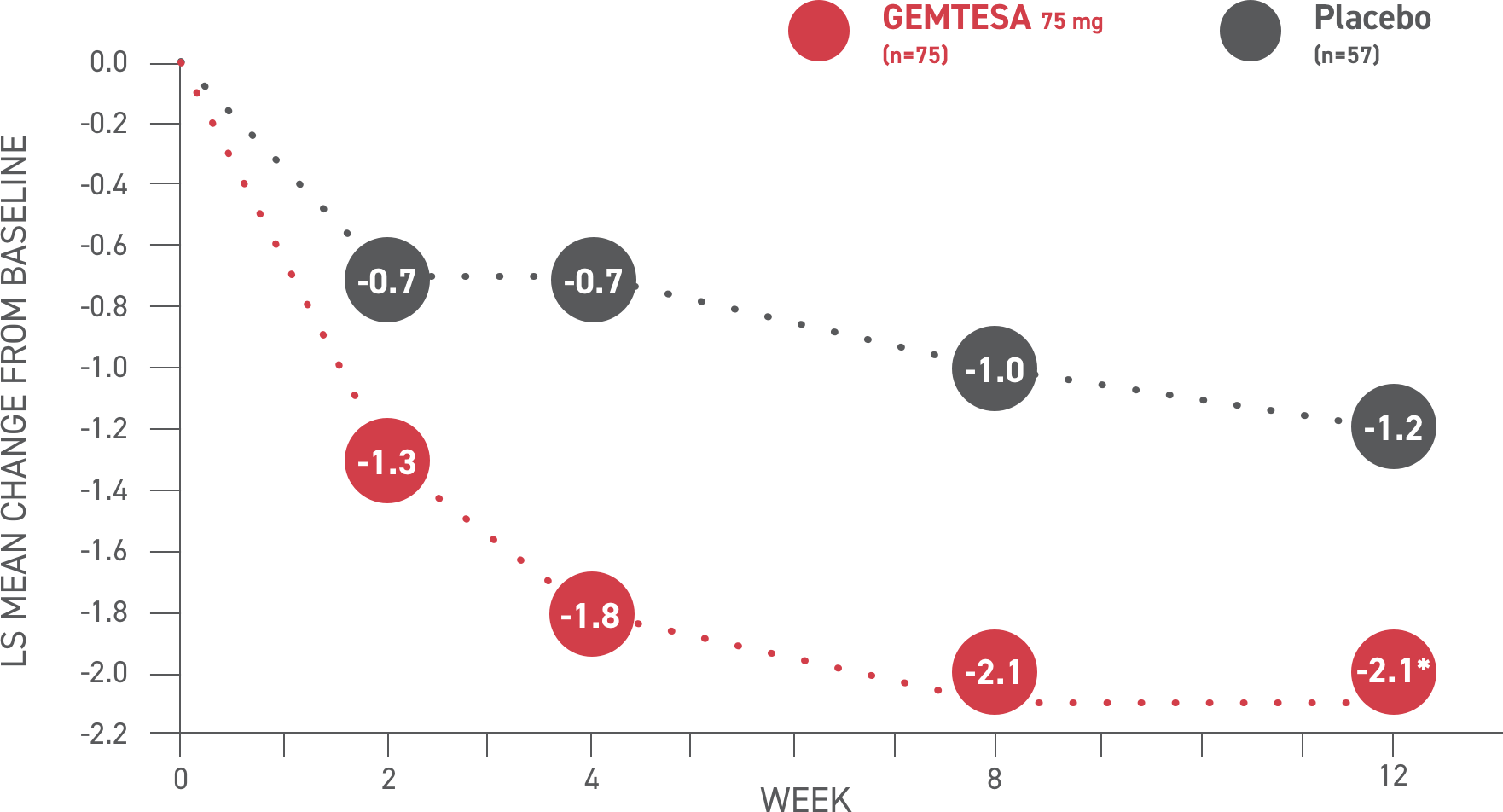
GEMTESA demonstrated significant reductions in average daily urgency episodes—need to urinate immediately—at 12 weeks vs placebo2*
*P<0.001 vs placebo.
Please see older adults data analysis above.
Urgency episodes2
Values
| Week | 2 | 4 | 8 | 12 |
|---|---|---|---|---|
| Placebo (n=405) | -0.8 | -1.0 | -1.2 | -1.4 |
| GEMTESA 75mg (n=403) | -1.4 | -1.7 | -1.8 | -2.0 |
GEMTESA demonstrated significant reductions in average daily urgency episodes—need to urinate immediately—at 12 weeks vs placebo2*
*P<0.01 vs placebo.
Please see older adults data analysis above.
Urgency episodes2
Values
| Week | 2 | 4 | 8 | 12 |
|---|---|---|---|---|
| Placebo (n=405) | -0.8 | -1.0 | -1.2 | -1.4 |
| GEMTESA 75mg (n=403) | -1.4 | -1.7 | -1.8 | -2.0 |
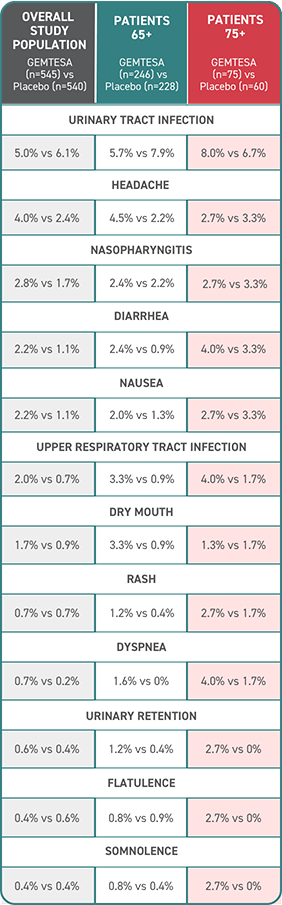
Established safety of GEMTESA for older adults at 12 weeks1,2
Adverse events (AEs) with an incidence of ≥2% compared with and exceeding placebo up to 12 weeks1,2
Overall Study
Population
Patients 65+
Patients 75+
GEMTESA
(n=545)
Placebo
(n=540)
GEMTESA
(n=246)
Placebo
(n=228)
GEMTESA
(n=75)
Placebo
(n=60)
URINARY TRACT
INFECTION
5.0%
6.1%
5.7%
7.9%
8.0%
6.7%
HEADACHE
4.0%
2.4%
4.5%
2.2%
2.7%
3.3%
NASOPHARYNGITIS
2.8%
1.7%
2.4%
2.2%
2.7%
3.3%
DIARRHEA
2.2%
1.7%
2.4%
0.9%
4.0%
3.3%
NAUSEA
2.2%
1.1%
2.0%
1.3%
2.7%
3.3%
UPPER RESPIRATORY
TRACT INFECTION
2.0%
0.7%
3.3%
0.9%
4.0%
1.7%
DRY MOUTH
1.7%
0.9%
3.3%
0.9%
1.3%
1.7%
RASH
0.7%
0.7%
1.2%
0.4%
2.7%
1.7%
DYSPNEA
0.7%
0.2%
1.6%
0%
4.0%
1.7%
URINARY RETENTION
0.6%
0.4%
1.2%
0.4%
2.7%
0%
FLATULENCE
0.4%
0.6%
0.8%
0.9%
2.7%
0%
SOMNOLENCE
0.4%
0.4%
0.8%
0.4%
2.7%
0%
Safety results to consider for older adults assessed at 12 weeks2
- No overall differences in safety or effectiveness of GEMTESA have been observed between patients aged 65+ or 75+ and younger adult patients
- Incidence of cardiovascular-associated AEs, including hypertension (1.3%) and increased blood pressure (0%), was low for elderly patients aged 75+ taking GEMTESA vs placebo (3.3% and 1.7%, respectively)
- Rates of discontinuation due to AEs were low for patients aged 65+ and 75+ (1.6% and 4%, respectively)