PROVEN RESULTS WITH GEMTESA
Significant improvement in overactive bladder (OAB) symptom relief at 12 weeks
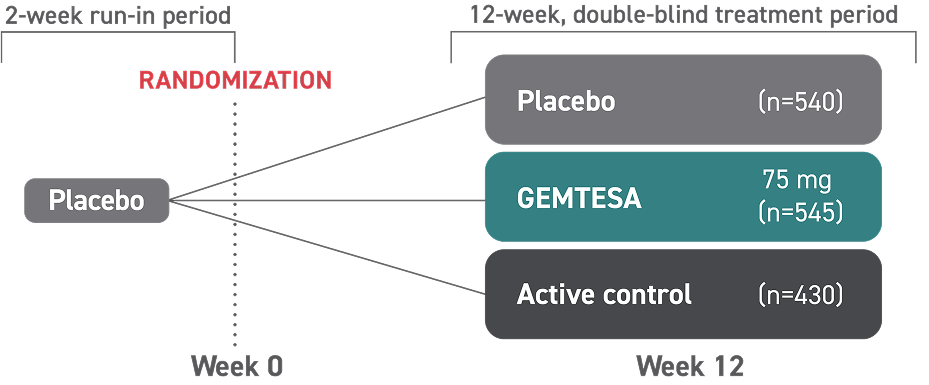
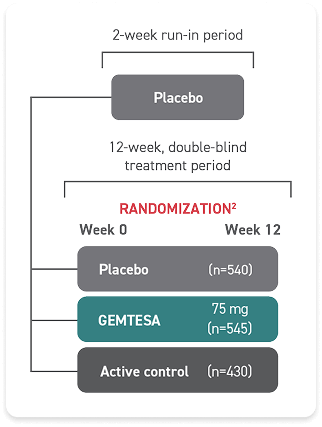
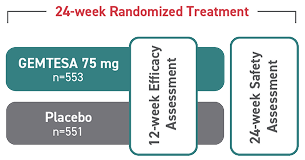
In a 12-week clinical study:
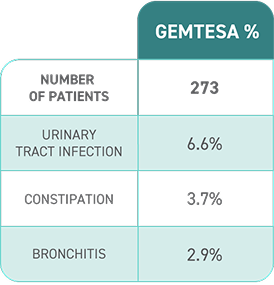
Additional clinical study results seen at 12 weeks:
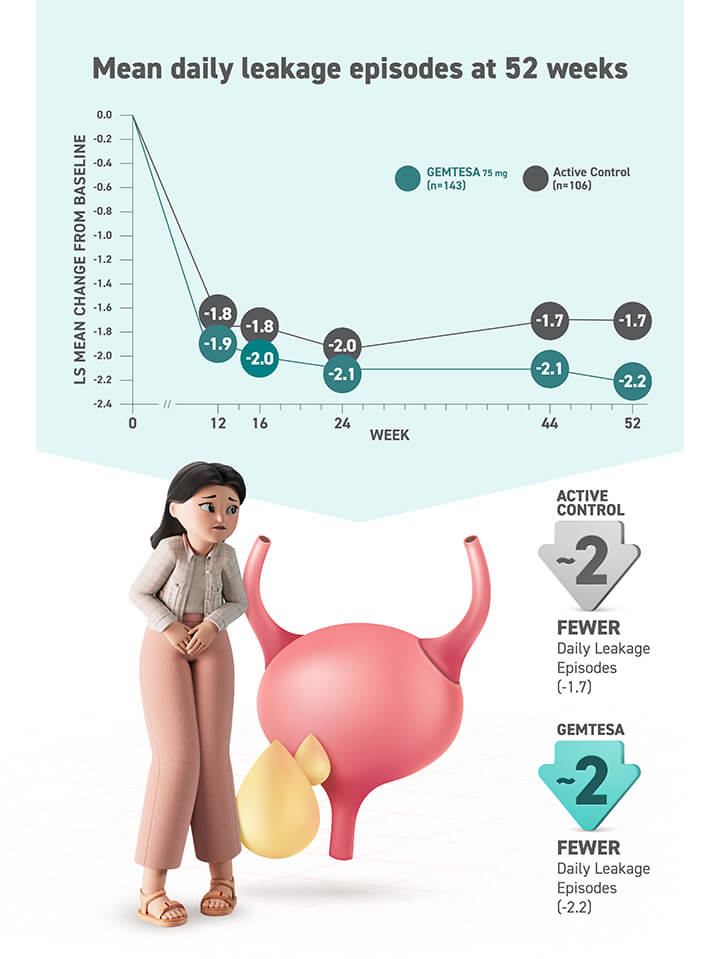
About half (52%) of people taking GEMTESA saw a 75% or greater reduction in daily leakage episodes vs 37% for people taking placebo.*
*403 people received GEMTESA and 405 people received placebo.
†526 people received GEMTESA and 520 people received placebo.

Fiction
Generic options treat OAB just as well as brand-name medicines.
Fact
GEMTESA is the only OAB treatment of its kind, and there is no generic substitute.
In fact, GEMTESA is the only OAB treatment of its kind with:
- Proven relief from all 3 OAB symptoms — leakage episodes, urgency, and frequency — in its label
- Proven efficacy and safety data in men also being treated for BPH (benign prostatic hyperplasia)
- No blood pressure warning in its label. At 12 weeks, people taking GEMTESA and those taking placebo had similar rates of hypertension (1.7% vs 1.7%, respectively), and increased blood pressure (0.7% vs 0.9%, respectively)
In a separate study of OAB in men being treated for BPH, rates of hypertension were 9.0% with GEMTESA vs 8.3% with placebo
Hear from Diane, a real patient moving beyond just coping with OAB
Since I started with GEMTESA, I have fewer OAB symptoms. I don't have to worry about where the bathrooms are all the time.
Diane is a real patient taking GEMTESA who has been compensated for her time.
Individual results may vary.
Transcript
[Text on screen] Diane’s Go-Getter Journey
My name is Diane, and my friends describe me as a connector and a mentor.
Overactive bladder, or OAB, had a major impact on my everyday life.
[Text on screen] Diane is a real patient taking GEMTESA who has been compensated for her participation.
I couldn't drink water like I should have. And I always had to make sure to go to the bathroom before I left the house.
And when I arrive at a restaurant, I would probably go to the bathroom first before sitting down and ordering food to make sure I could make it through the meal.
So, it permeated my life.
I remember one time, I was sitting out on my patio enjoying a lovely brunch with my girlfriends and all of a sudden I felt that got-to-go feeling. And I stood up and I just had an accident, a wetting accident.
And I was so mortified.
[Text on screen] Please see Important Safety Information included in this video.
Like many people, I thought these symptoms — like urgency — that got-to-go NOW feeling, frequency that got-to-go often feeling, and leakage or wetting accidents — were just a normal part of aging.
When I was struggling with OAB symptoms, I tried everything from Kegels to other medications, but I still didn't feel comfortable enough to wear pads less often or be too far from a bathroom.
That's when I finally asked my doctor about GEMTESA.
And let me tell you, I am so glad I did.
I have places to go and people to encourage with my go-getter attitude.
GEMTESA is a prescription medicine used to treat the following symptoms due to a condition called overactive bladder (OAB) in adults, and in adult males taking medicine for benign prostatic hyperplasia (BPH): leakage episodes, urgency, and frequency.
[Text on screen] GEMTESA is a prescription medicine used to treat the following symptoms due to a condition called overactive bladder (OAB) in adults, and in adult males taking medicine for benign prostatic hyperplasia (BPH): leakage episodes, urgency, and frequency.
Do not take GEMTESA if you are allergic to vibegron or any of the ingredients in GEMTESA.
[Text on screen] Do not take GEMTESA if you are allergic to vibegron or any of the ingredients in GEMTESA.
GEMTESA may cause serious side effects such as:
- inability to empty your bladder. Tell your doctor right away if this happens.
- an allergic reaction with swelling of the lips, face, tongue, or throat, with or without difficulty breathing and may be life-threatening. Stop using GEMTESA and get emergency medical help right away if you have any of these symptoms.
[Text on screen] GEMTESA may cause serious side effects such as:
- inability to empty your bladder. Tell your doctor right away if this happens.
- an allergic reaction with swelling of the lips, face, tongue, or throat, with or without difficulty breathing and may be life-threatening. Stop using GEMTESA and get emergency medical help right away if you have any of these symptoms.
My doctor told me about GEMTESA, and about the most common side effects including headache, urinary tract infection, nasal congestion, sore throat or runny nose, diarrhea, nausea, and upper respiratory tract infection.
[Text on screen] Most common side effects may include headache, urinary tract infection, nasal congestion, sore throat or runny nose, diarrhea, nausea, and upper respiratory tract infection.
I didn't know OAB was chronic, but I do now.
Like any chronic condition, it's important to stick to your treatment to see continued results.
My doctor told me I should start to see results on GEMTESA after 12 weeks of consistent use, so I made sure to take GEMTESA every day.
He also told me that GEMTESA isn't an overnight fix.
[Text on screen] Individual results may vary.
There are no overnight fixes.
You have to be consistent with taking GEMTESA to see results.
I've been on GEMTESA for three years now and I can't imagine going back.
Since I started with GEMTESA, I have fewer OAB symptoms.
I don't have to worry about where the bathrooms are all the time.
I wish I'd known about GEMTESA sooner, but now it's my mission to help others know there are options for treating OAB.
I think there's definitely a stigma, but I hope to change that because I feel that if I'd talked to other women sooner, I may not have needed to cope or suffer in silence for so long.
I want you to go do something other than just cope with OAB symptoms and talk to your doctor.
[Text on screen] Putting the “go” in Go-Getter!
[logo] GEMTESA® (vibegron) 75 mg tablets
Do more than just cope with OAB symptoms. Talk to your doctor about treatment options today.
[logo] TIME TO GO
Full Product Information can be found at www.GEMTESA.com/PI
What is GEMTESA?
GEMTESA is a prescription medicine used to treat the following symptoms due to a condition called overactive bladder in adults, and in adult males taking medicine for benign prostatic hyperplasia (BPH):
- urge urinary incontinence: a strong need to urinate with leaking or wetting accidents
- urgency: the need to urinate right away
- frequency: urinating often
It is not known if GEMTESA is safe and effective in children.
IMPORTANT SAFETY INFORMATION
Do not take GEMTESA® (vibegron) if you are allergic to vibegron or any of the ingredients in GEMTESA.
Before you take GEMTESA, tell your doctor about all your medical conditions, including if you have liver problems; have kidney problems; have trouble emptying your bladder or you have a weak urine stream; take medicines that contain digoxin; are pregnant or plan to become pregnant (it is not known if GEMTESA will harm your unborn baby; talk to your doctor if you are pregnant or plan to become pregnant); are breastfeeding or plan to breastfeed (it is not known if GEMTESA passes into your breast milk; talk to your doctor about the best way to feed your baby if you take GEMTESA).
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
What are the possible side effects of GEMTESA?
GEMTESA may cause serious side effects including:
- inability to empty your bladder (urinary retention). GEMTESA may increase your chances of not being able to empty your bladder, especially if you have bladder outlet obstruction or take other medicines for treatment of overactive bladder. Tell your doctor right away if you are unable to empty your bladder.
- angioedema. GEMTESA may cause an allergic reaction with swelling of the lips, face, tongue, or throat, with or without difficulty breathing and may be life-threatening. Stop using GEMTESA and get emergency medical help right away if you have symptoms of angioedema or trouble breathing.
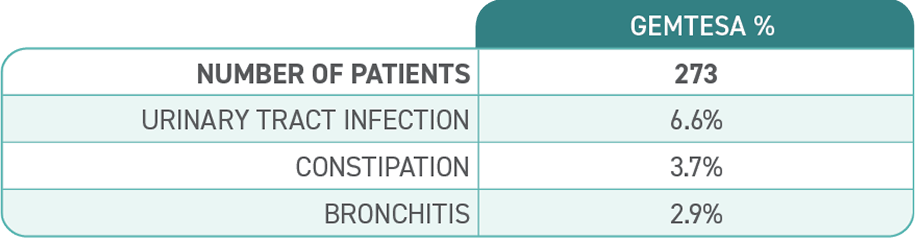
The most common side effects of GEMTESA include headache, urinary tract infection, nasal congestion, sore throat or runny nose, diarrhea, nausea and upper respiratory tract infection. These are not all the possible side effects of GEMTESA. For more information, ask your doctor or pharmacist.
These are not all the possible side effects of GEMTESA. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Please see full Prescribing Information at www.GEMTESA.com/PI.